Collective cell behavior …
… in tissue injury and inflammation
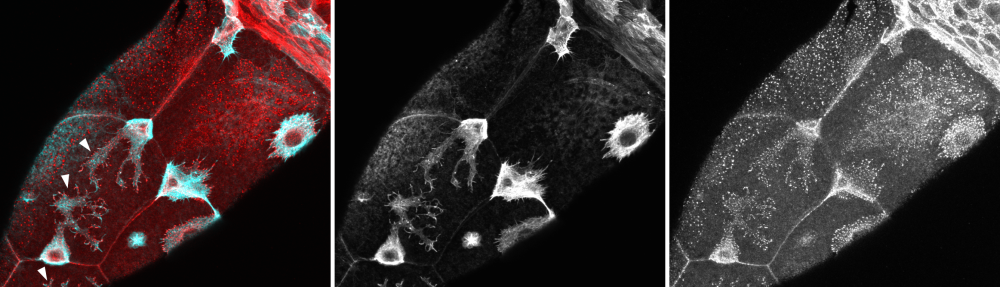
The restoration of homeostasis after tissue damage relies on proper spatial-temporal control of distinct cell behaviors. We are particularly interested in how the intricate balance between proliferation and cell death is controlled. In Drosophila imaginal discs, this balance is coordinated by the conserved JNK/AP-1 and JAK/STAT signaling pathways. We described that cells at the centre of inflammatory wounds undergo a JNK-induced cell cycle arrest in G2. Surprisingly, this reversible arrest induces senescence features, such as expression of cytokine (SASP), resistance to apoptosis and upregulation of cellular defence pathways. Importantly, on short timescales it promotes tissue repair. However, during chronic inflammation, it establishes a chronic wound phenotype and contributes to the oncogenic potential of a tumor microenvironment. We are currently investigating how these senescent cells adapt cellular processes, metabolic pathways and epigenetic modifications for their important function in tissue repair. Of course, we are also interested in how cells in the periphery of the wound proliferate and mediate tissue regeneration after damage. We try to better understand, for example, what the role of the JAK/STAT pathway may be in promoting anabolic growth and cell cycle adaptions that allow for fast proliferation in regenerating domains.
… in the presence of aberrant cells
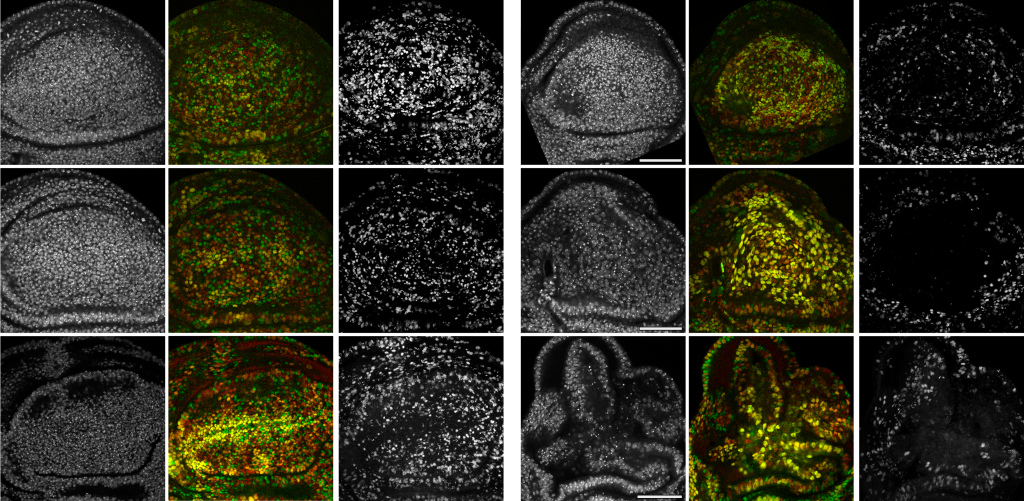
Because of their exposed position, epithelia are susceptible to mutagenesis. As a consequence, genetically altered or transformed cells arise at a constant rate in epithelial tissues. Typically, elimination of these cells from an organism has been attributed to immunosurveillance mechanisms. More recently, tissue-intrinsic surveillance mechanisms – such as cell-cell competition – driving elimination of aberrant cells have been described. These processes share two striking feature: (1) the elimination of aberrant cells is driven by surrounding wild type cells and (2) elimination requires cell contact between normal and eliminated cells. We established a paradigm, in which aberrantly-specified and RasV12-transformed cells are detected by a tissue intrinsic surveillance mechanism that we now call interface surveillance. We are now investigating which cell biological mechanisms and signaling pathways mediate recognition and elimination of aberrantly specified cells. Surprisingly we found that Robo receptors and other cell surface molecules previously implicated in axon guidance play a role in detection of aberrant cells. A mismatch in expression levels between neighboring cells induces a downstream signaling cascade involving JNK activation that removes abnormal cells. Further revealing these cellular recognition and elimination strategies is crucial to understand how transformed cells may evade these intra-epithelial tumor suppression mechanisms and how these strategies may become therapeutic targets.
… in tissue morphogenesis
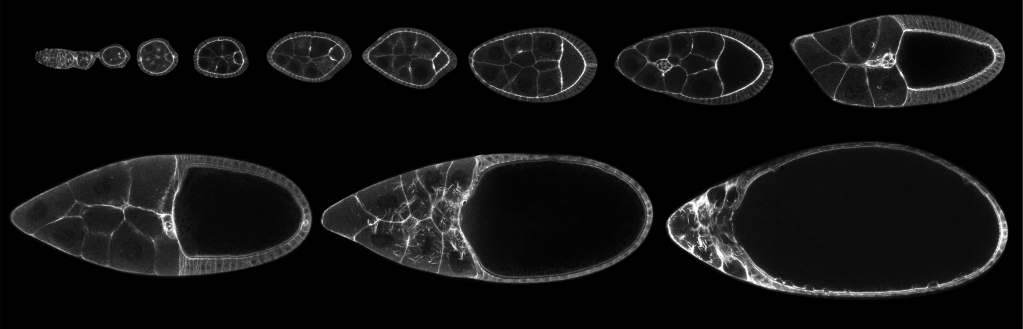
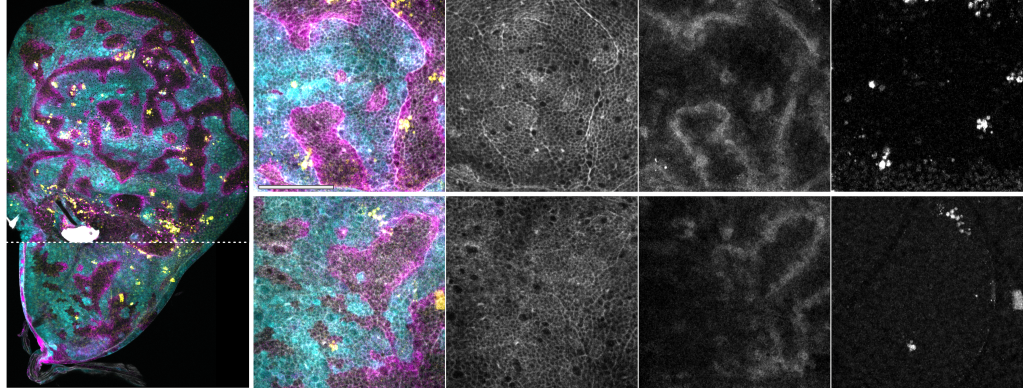
We are also interested in tissue morphogenesis, particularly in how cells, and different cell lineages, cooperate to organize biological architectures and shapes. Currently, we are focusing on the interaction between somatic cells and germline cells during the development of the Drosophila egg chamber (oogenesis). In many species, oocytes develop within a germline cyst, which includes the developing oocyte, nurse cells and the surrounding somatic epithelium. Nurse cells provide nutrients for oocyte growth and undergo programmed cell death and removal at the end of oogenesis. The surrounding cells of the somatic epithelium differentiate to either support oocyte maturation or facilitate nurse cell removal. Tight morphogenetic coordination between the surrounding somatic epithelium, nurse cells and the oocyte is essential to manage the dramatic volume changes that occur during oocyte growth. Our previous research has shown that the dynamic expression of the transcriptional regulator Eyes absent (Eya) in the somatic epithelium plays a crucial role in establishing differential cell-cell affinity between soma and germline cells throughout egg chamber development. The mechanical forces resulting from this process redistribute somatic cells across the germline surface. This modulates oocyte growth and ensures alignment of oocyte and nurse cells with their corresponding somatic counterparts. This mechanism is crucial for the self-organization of tissues during oogenesis, highlighting the importance of differential affinity between cell lineages in the assembly of complex inter-lineage functional units. We are currently investigating the cell-biological basis of differential soma-germline affinity and the molecular mechanisms within somatic and germline cells that maintain the mechanical equilibrium of egg chambers during different phases of oocyte growth. Our focus is on understanding the regulation of differential adhesion through transmembrane adhesion receptors, the modulation of cortical tension via actomyosin networks, and the dynamic remodeling of cell protrusions such as lamellipodia and filopodia during soma-germline interactions.